Abstract : The process of high temperature liquid phase catalytic oxidation of p-xylene to terephthalic acid requires the use of a Co-Mn-Br ternary composite catalyst. Yizheng Chemical Fiber Co., Ltd. Chemical plant catalyst recovery system introduced the advanced technology of the United States DuPont company, the use of recovery reagents to make most of the cobalt and some of the mother liquor precipitation, and then separated by butterfly centrifugal centrifuge. The equipment principle, process and reaction mechanism of the process are briefly described, and simple economic and technical comparisons are made. The results show that the process can reduce the use of fresh catalyst by nearly 50%, saving production costs of 8 million yuan each year, and reducing the emission of heavy metal pollutants.
Key words: Terephthalic acid;Co-Mn-Br three-way catalyst;recovery
Terephthalic acid (PTA) is an important raw material for the production of polyester fibers and plastics. It is mainly used to synthesize polyester intermediates—PET, from para-xylene (PX). High temperature liquid catalytic oxidation. The reaction uses acetic acid (HAc) as solvent, cobalt acetate and manganese acetate as catalysts, bromide as accelerator, air as oxidant, and gas-liquid-solid three-phase heat release at 180-240° C. and 10-25 MPa. Reaction [1]. The cobalt in the catalyst is a rare metal and the price is high. In the early stage of industrialization of the PTA plant, higher manganese-cobalt ratios were generally used to reduce the amount of cobalt in order to reduce costs. However, since the catalytic activity of manganese is much lower than that of cobalt, and the by-products that affect the efficiency of the filter are easily formed during the reaction, the product yield is low and the color is poor. For this reason, the ratio of manganese to cobalt has to be reduced, which in turn makes it impossible to reduce the production cost of PTA. A part of the catalyst added to the system goes through the crude terephthalic acid (TA) into the refining system, and the other part is pumped through the mother liquor into the residue treatment system. Discharging with the residue not only causes environmental pollution, but also the loss of noble metal cobalt increases the production cost[2] . It has been reported that the PTA catalyst recycling technology includes incineration recovery method, return oxidation method, ion exchange method, electrodialysis method, and solvent extraction method [3]. Jiangsu Yizheng Chemical Fiber Co., Ltd. Chemical Plant Catalyst Recovery System introduced the advanced technology of DuPont, which uses recovery reagents to precipitate most of the cobalt and part of the manganese in the mother liquor. Then it is separated by a butterfly centrifuge and recovered. The catalyst can be returned directly to the system for use. The system is relatively advanced in the current PTA catalyst recycling technology and can recover nearly 90% of cobalt ions and about 75% of manganese ions, reducing the use of nearly 50% of fresh catalyst.
1 Process and Process Principle
1.1 Process flow
The part of the filtrate extracted by the oxidation filter is no longer sent to the residue treatment system and is directly sent to the filtrate buffer tank. After being pumped and mixed with oxalic acid, the filtrate is precipitated and separated in a butterfly-type centrifuge. Precipitation of the catalyst metal in the mother liquor causes the precipitation of bromine, and the free bromine causes stress corrosion in the downstream equipment. To this end, a certain proportion of lye is added to the centrifuge feed to prevent corrosion. After the separation of the overflow solution to the residue treatment system to recover the solvent, remove impurities. The cobalt oxalate and manganese oxalate obtained after centrifugation are recovered in the catalyst storage tank, returned to the mother liquor together with the fresh cobalt manganese catalyst and HBr, and returned to the oxidation reactor to recover the catalyst metal, reduce the production cost, and reduce the environmental pollution. purpose.
Catalyst recovery process shown in Figure 1.
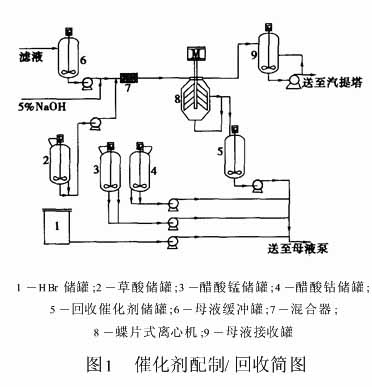
1.2 Process Principle 10% (mass fraction) oxalic acid solution from oxalic acid storage tank is mixed with mother liquor in a certain ratio to produce cobalt oxalate and manganese oxalate precipitate. Since oxalic acid has strong corrosiveness, in order to prevent severe corrosion of equipment, the amount of oxalic acid added should be strictly controlled. The addition of oxalic acid can only precipitate approximately 90% of the metal ions to prevent the excess oxalic acid from reacting with the equipment metal. The molar ratio of oxalic acid to the catalyst metal is approximately 0 9:1.
Since cobalt precipitates preferentially over manganese, nearly all cobalt can be recovered, and the remaining oxalic acid can recover most of the manganese. As a result of the precipitation reaction between the catalyst metal and oxalic acid in the mother liquor, free bromide ions are precipitated from the mother liquor and cause stress corrosion to the downstream equipment. NaOH can be added to the mother liquor in advance to prevent corrosion. However, excessive Na+ ions enter the mother liquor with the recovered catalyst, and are recycled back to the oxidation reactor to deactivate the catalyst. Under the premise of controlling the concentration of Na+ in the mother liquor, the molar ratio of alkali to bromine should be controlled to at least 15:1 for safety reasons of equipment.
2 Equipment Principles and Control Points
2.1 Equipment Principle
Butterfly-type centrifuge is a low-voltage equipment, stainless steel, diameter 762mm, drum is equipped with about 100 fins, fin gap of about 1mm, speed 3000r/min. Butterfly-type centrifuges are used to separate oxalate precipitates and crude terephthalic acid solids from the catalyst metal in the mother liquor, and approximately 95% of the solids in the mother liquor can be recovered.
The mother liquor and oxalic acid are mixed thoroughly by a mixer, and the catalyst metal in the mother liquor generates oxalate, which is rapidly precipitated into a butterfly-type centrifuge and recovered as a slurry. The mother liquor enters the inlet of the centrifuge and passes through the center of the centrifuge. A small hole is formed in the butterfly piece, and the mother liquid flows through the hole to the gap of the butterfly piece. Under the action of centrifugal force, the heavy liquid settles along the inclined surface of the butterfly and moves to the inner wall of the drum. It is collected under the rotor of the butterfly and discharged from the outlet nozzle of the heavy liquid at the edge of the centrifuge; and the light liquid follows the slope of the butterfly. Moving up [4], it rises in the middle of the centrifuge and is collected and discharged from the overflow system at the top of the light liquid outlet. If the solids content of the mother liquor is too high, it can clog the equipment. In order to prevent blockage of the slurry in the underflow line, the equipment has a flushing procedure. To achieve the goal of recovering the catalyst, the concentration of the recovered catalyst is controlled by the underflow meter. About 90% of the slurry is recycled back to the bottom of the centrifuge, and the centrifugal separation process is repeated to finally form an underflow product with a solid content of about 5%. The schematic diagram of catalyst centrifugal recovery is shown in Figure 2.

2.2 Control Points
As a high-speed rotating equipment with a speed of 3000r/min, the butterfly-type centrifuge has a series of monitoring instruments for monitoring equipment vibration, main motor current, shaft temperature, motor winding temperature, valve position status and other parameters. The above operating parameters should be closely monitored, because changes in the operating parameters not only show the safe operating conditions of the equipment, but also indicate whether the recovery separation effect is good or bad. It is necessary to strictly control the ratio of oxalic acid, alkali and mother liquor in order to fully recover the catalyst metal without corrosion of the equipment. During the normal feeding operation process, the vibration amplitude of the equipment was found to be lower than normal. Combined with the daily analysis results, it can be analyzed to determine whether the gap between the blades is blocked and the separation efficiency is reduced. The butterfly centrifuge should be alkali-washed or alkaline foamed in time.
Autumn & Winter Style Nails,Denim False Nails Kits,3D Sweater False Nails,Yellow Fake Nails
Zhong Shan Senboma Artware Co.,Ltd , https://www.senbomanails.com